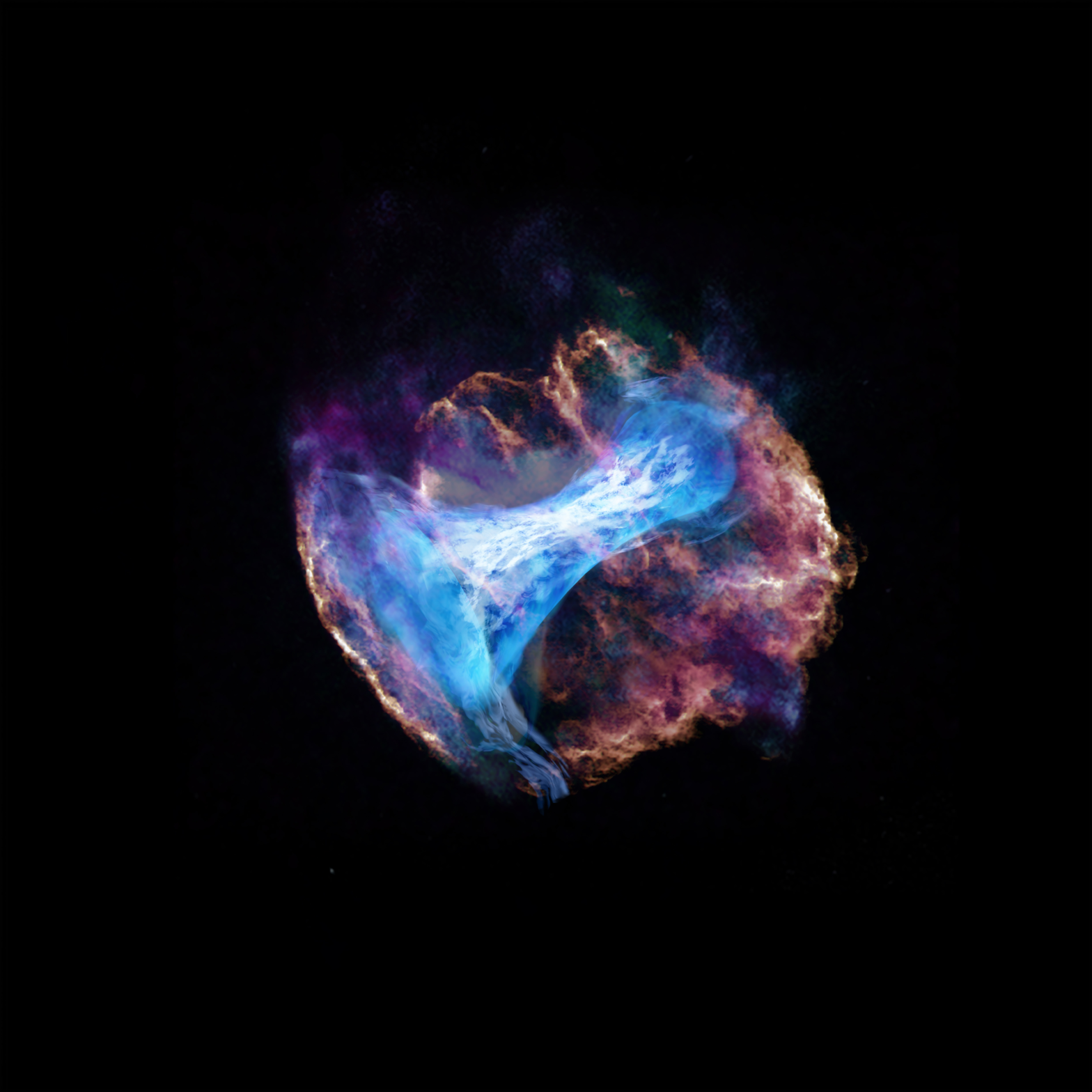
The X-ray mission XRISM has uncovered new clues to how certain chemical elements ―specifically those with odd atomic numbers (odd number of protons), such as chlorine and potassium― were created in the Universe. By observing the famous supernova remnant Cassiopeia A, XRISM detected clear X-ray signals from these elements for the first time.
Chlorine and potassium are essential for life on Earth, and this discovery suggests that their abundances could be due to the insides of massive stars being far more active and mixed than previously believed before they explode.
Background: Where did these elements come from?
When the Universe was born 13.8 billion years ago, it contained only the lightest elements― mainly hydrogen and helium. All heavier elements, such as carbon, oxygen, and iron, were formed later inside stars. When particularly massive stars end their lives in cosmic explosions known as supernovae, they scatter these newly created elements into space, providing the raw material for planets and eventually for life.
However, some elements― especially odd atomic number elements like chlorine (number of protons, Z = 17) and potassium (Z = 19) ― have been difficult to explain.
Standard models suggest that stars do not produce large amounts of these elements, yet they are relatively abundant in the Universe. Because these elements are vital for biological processes, understanding their cosmic origin is a key scientific challenge.

XRISM’s high-resolution spectrometer, Resolve, can distinguish extremely faint X-ray signals that earlier instruments could not detect. Using this capability, XRISM clearly identified chlorine and potassium emission coming from Cassiopeia A (Fig. 2). A particularly important finding is that these elements are concentrated in regions where oxygen is also abundant. This is surprising because, in the traditional “onion-like” model of a massive star’s interior, each layer contains different elements, and odd atomic number elements should not form efficiently in oxygen-rich layers.
The new observations point to a different scenario.
Before the star exploded, materials from different layers ―such as oxygen and neon― must have mixed violently. This mixing enables new nuclear reactions that can produce odd atomic number elements much more efficiently than previously expected.
What triggered this mixing?
Possible explanations include strong convection inside the star, rapid rotation, or interactions in a binary system. Although the main mechanism is still uncertain, XRISM’s measurements provide strong evidence that powerful internal motions occurred before the explosion.
Why this matters
This discovery brings scientists much closer to solving a long-standing mystery:
How were odd atomic number elements ―important both for planets and for life― created in significant amounts across the Universe?
By detecting these elements and identifying where they formed inside the star, XRISM offers a major step forward in understanding the chemical evolution of the Universe.
What comes next
Although this study shows that chlorine and potassium were efficiently produced in parts of Cassiopeia A, it is still unclear whether this mechanism alone is enough to explain their abundance throughout the Universe.
In the coming years, XRISM will observe other regions of Cassiopeia A and additional supernova remnants to better determine how much of these elements massive stars can produce.
These observations will help scientists refine the “cosmic recipe” that shaped the chemical makeup of the Universe.
Paper Information
Journal: Nature Astronomy
Title: Chlorine and Potassium Enrichment in the Cassiopeia A Supernova Remnant
Authors: XRISM Collaboration
DOI: 10.1038/s41550-025-02714-4